10.20986/revesppod.2025.1717/2024
NOTA CLÍNICA
Topical imiquimod 5 % in the treatment of recalcitrant plantar warts. Case report
Imiquimod 5 % tópico en el tratamiento de verrugas plantares recalcitrantes. Caso clínico
Manuel Menéndez-Salas1
Sara García-Oreja1
José Luis Lázaro-Martínez1
Francisco Javier Álvaro-Afonso1
1Clínica Universitaria de Podología. Universidad Complutense de Madrid, España
Abstract
Plantar warts are lesions caused by the Human Papilloma Virus (HPV) and affect both children and adults. There is a wide variety of treatments depending on the extension, time of evolution of the lesion and previous treatments. Imiquimod is an immune response modulator drug approved by the Spanish Agency for Medicines and Medical Devices for the treatment of genital warts, basal cell carcinoma and actinic keratosis in adults. The aim of this work is to show a clinical case of multiple and recalcitrant plantar warts treated with a topical antiviral drug, whose active ingredient is Imiquimod 5 %. The patient was 26 years old, with multiple HPV mosaic plantar warts, positive for biotypes 2 and 27, of 3 years of evolution, with pain on walking, who came to the clinic without having reported improvement with topical treatments. After 8 weeks applying Imiquimod 5 % cream 3 times a week, total resolution of the lesions was observed, without pain or adverse effects, so Imiquimod 5 % cream can be an effective and safe alternative in the treatment of HPV plantar warts.
Keywords: Plantar wart, HPV, papillomavirus, treatment, antivirals
Resumen
Las verrugas plantares son lesiones producidas por el virus del papiloma humano (VPH) y afectan tanto a la población infantil como a la adulta. Existe una gran variedad de tratamientos en función de la extensión, tiempo de evolución de la lesión y los tratamientos previos. El imiquimod es un fármaco modulador de la respuesta inmunitaria aprobado por la Agencia Española del Medicamento para el tratamiento de verrugas genitales, carcinoma basal y queratosis actínica en adultos. El objetivo de este trabajo es mostrar un caso clínico de verrugas plantares múltiples y recalcitrantes en las que se aplicó como tratamiento un antiviral tópico, cuyo principio activo es el imiquimod al 5 %. Se trata de una paciente de 26 años, que presentaba múltiples verrugas plantares en mosaico por VPH, positivas a los biotipos 2 y 27, de 3 años de evolución, con dolor a la deambulación, que acude a consulta sin haber referido mejoría con distintos tratamientos tópicos. Tras 8 semanas aplicando imiquimod al 5 % crema 3 veces a la semana, se observó una resolución total de las lesiones, sin presentar dolor ni efectos adversos, por lo que el imiquimod 5 % crema puede ser una alternativa eficaz y segura en el tratamiento de verrugas plantares por VPH.
Palabras clave: Verruga plantar, VPH, papilomavirus, tratamiento, antivirales
Corresponding author
Sara García Oreja
sagarc14@ucm.es
Received: 04-11-2024
Accepted: 27-01-2025
Introduction
Warts are very common skin lesions on the soles of the feet caused by the Human Papillomavirus (HPV) and affect both children and adults(1). They are characterized by the appearance of hyperkeratosis and hemorrhagic punctate, the loss of skin dermatoglyphs, and pain(2). According to some studies, around 40% of the population is infected with HPV, and between 7% and 12% develop plantar warts². Currently, the annual incidence is 14%(2). This rate varies depending on factors such as age, sex, race, and health status, among others(2).
More than 150 types of human papillomavirus (HPV) have been identified, most of which are benign(1). Subtypes 1, 2, 4, 10, 27, and 57 are the most frequently found in foot warts(1). Infection occurs through contact with viral particles, which can be direct, by coming into contact with a plantar wart, or indirect, through body fluids, shoes, or infected surfaces, among others¹. Other factors that predispose to the appearance of plantar warts include hyperhidrosis, the presence of trauma and lacerations, having had a wart previously, immune system disorders, and stress(1).
There are multiple treatments with very heterogeneous results, some more invasive than others(3,4,5). This may be because, unlike other viruses, HPV does not present antigens to the immune system, so no immune response is generated, and no inflammatory process is triggered(1). The most aggressive treatments are usually the fastest and most effective, while conservative treatments, such as cryotherapy, tend to be slower(5,6,7).
Generally, the first therapeutic step is conservative treatments, such as 70% salicylic acid, cryotherapy (liquid nitrogen), the nitric acid and zinc complex (Verrutop®), followed by the magistral formula of cantharidin, podophyllin, and salicylic acid (CPA), and finally surgical procedure(3,8). A recent systematic review revealed that different treatments have variable cure rates, with the CPA formula showing the highest cure rate of all observed, at 97.82%(3).
Currently, in Spain, the only topical antiviral authorized for the treatment of plantar warts caused by HPV is the 5-Fluorouracil + Salicylic Acid complex (Verrucután®). It is a topical antineoplastic and antimetabolite drug that blocks DNA and RNA synthesis, preventing cell replication?.
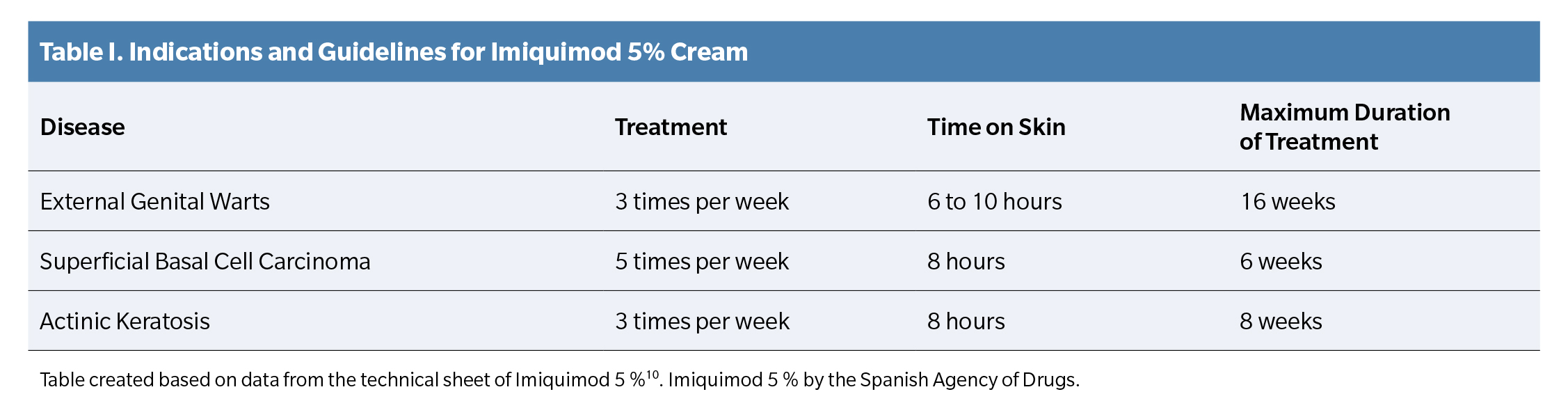
Imiquimod 5% cream is an immune response modulator currently approved by the Spanish Agency for Medicines and Health Products (AEMPS) for the treatment of genital warts, superficial basal cell carcinoma, and actinic keratosis in adults (Table 1) (10).
Imiquimod has been shown to have antiviral and antitumor effects in animal models, although it does not show direct antiviral or antitumor activity in vitro. The exact mechanism of action of Imiquimod in humans remains unknown. However, in general terms, its application stimulates the production of various pro-inflammatory cytokines, especially interferon-alpha (IFN-α) and tumor necrosis factor-alpha (TNF-α), which strengthens the cellular immune response. Thus, Imiquimod promotes a local immune response through the induction of cytokines(11).
The objective of this work is to present a clinical case of multiple and recalcitrant plantar warts in which a topical antiviral, whose active ingredient is 5% Imiquimod, was applied as treatment with good resolution of the case.
Case report
A 26-year-old woman first visited the Chiropodology and Surgery Service of the Clínica Universitaria de Podología (Madrid, Spain) in the last week of May 2024, with a 3-year history of mosaic plantar warts in the forefoot area. She reported having been put on treatments by her dermatologist, with which she had not experienced improvement but rather worsening and spread of the warts. The lesions had been previously treated with cryotherapy (cryotherapy applications every 6 months) and combined treatment with 5-Fluorouracil + Salicylic Acid (Verrucután®) simultaneously every night, wrapping the area in transparent paper (for almost 1 year). Due to the lack of improvement, she was prescribed the CPA formula, also without reporting any improvement. The patient reported no food or drug allergies and had no relevant personal medical history. She only reported taking the contraceptive pill as her regular drug.
Upon inspection, multiple mosaic plantar warts were observed in the plantar area of the metatarsal heads and at the level of the third and fourth toes of the right foot (Figure 1). Additionally, excessive sweating was observed throughout the foot.
The patient reported pain while walking and having to modify her gait to avoid the pain disabling her in her daily life. Significant irritation and skin flaking due to previous chemical treatments were observed. The patient reported being psychologically affected due to the lack of improvement over the past few years and the limitations she faced due to the increasing pain she had experienced in recent years (Figure 1).
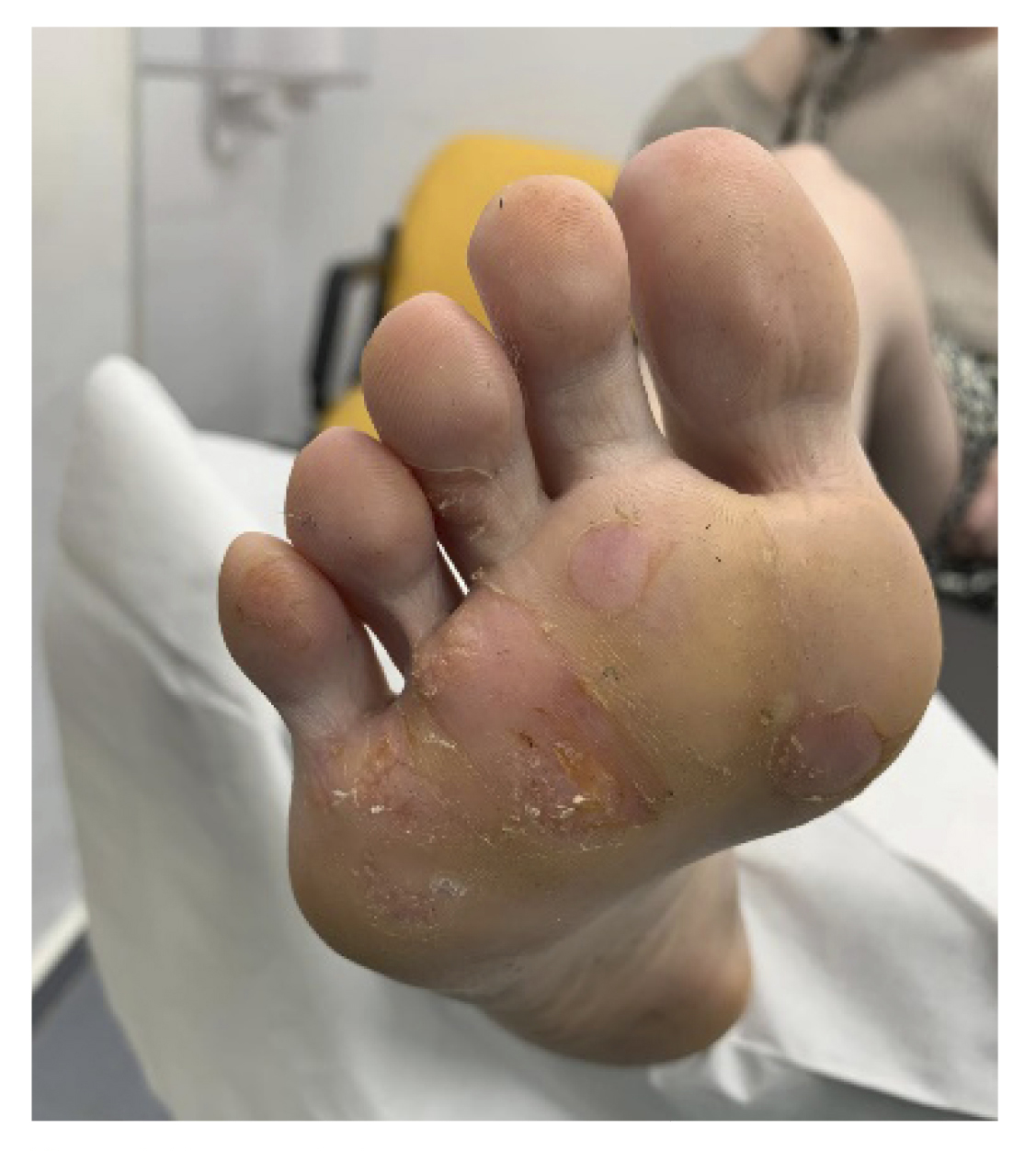
Figure 1. First consultation.
Although clinical signs were clear, a PCR (polymerase chain reaction) sample was taken to determine the virus biotype and, based on the biotype, prescribe the appropriate treatment. Daily washing with Germisdin® as an antiseptic gel was prescribed to control microbial flora and sweating until the results were delivered, scheduled for 2 weeks later.
Twenty days later, the patient returned for the delivery of culture results, showing improvement in the perilesional skin after the prescribed treatment in the previous consultation.
The PCR result came out POSITIVE for HPV for 2 different biotypes, 2 and 27, two of the most frequently isolated biotypes in plantar warts, and biotype 27 has been associated in former studies with recalcitrant plantar warts(4,12). After having undergone multiple previous treatments without good results, treatment with Imiquimod (Inmunocare ®) 50 mg/g cream was proposed. This treatment, as previously mentioned, is authorized by the AEMPS for the treatment of anogenital warts but is not indicated in its technical sheet for the treatment of plantar warts(10). Therefore, the patient was informed, and her informed consent statement was collected, following the latest modification of July 27th, 2013, of Royal Decree 1015/2009, of June 19th, which regulates the availability of drugs in special situations, in which the use of approved medicines is regulated when there is a need to use them under conditions different from those authorized, eliminating the need for prior individual authorization in each case by the AEMPS, and reinforcing the responsibility of healthcare centers, patient information, and the monitoring of their use(13).
Therefore, an occlusive application every 48 hours (3 applications per week) was prescribed, as indicated in the treatment of other types of warts in the drug’s technical sheet¹? (Table 1), and a review was scheduled for one week later.
In the following review (Figure 2. Week 2), we observed improvement in all lesions, still presenting hemorrhagic punctate in some lesions (Figure 3). The patient reported no adverse effects or secondary wounds from the application of the medication, in addition to reporting the absence of painful symptoms for the first time in years. Therefore, the treatment was maintained with the same regimen for 2 more weeks.
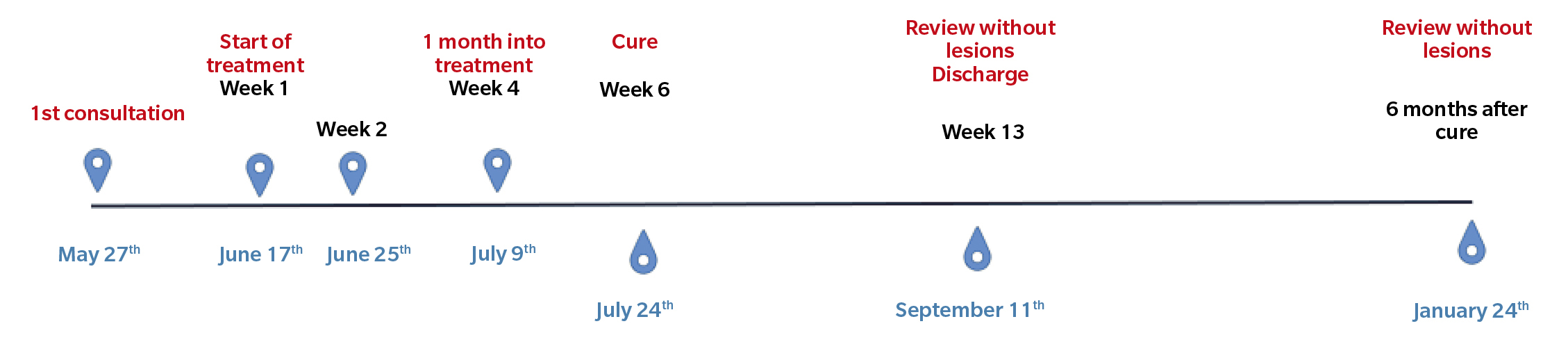
Figure 2. Chronology of the clinical case.
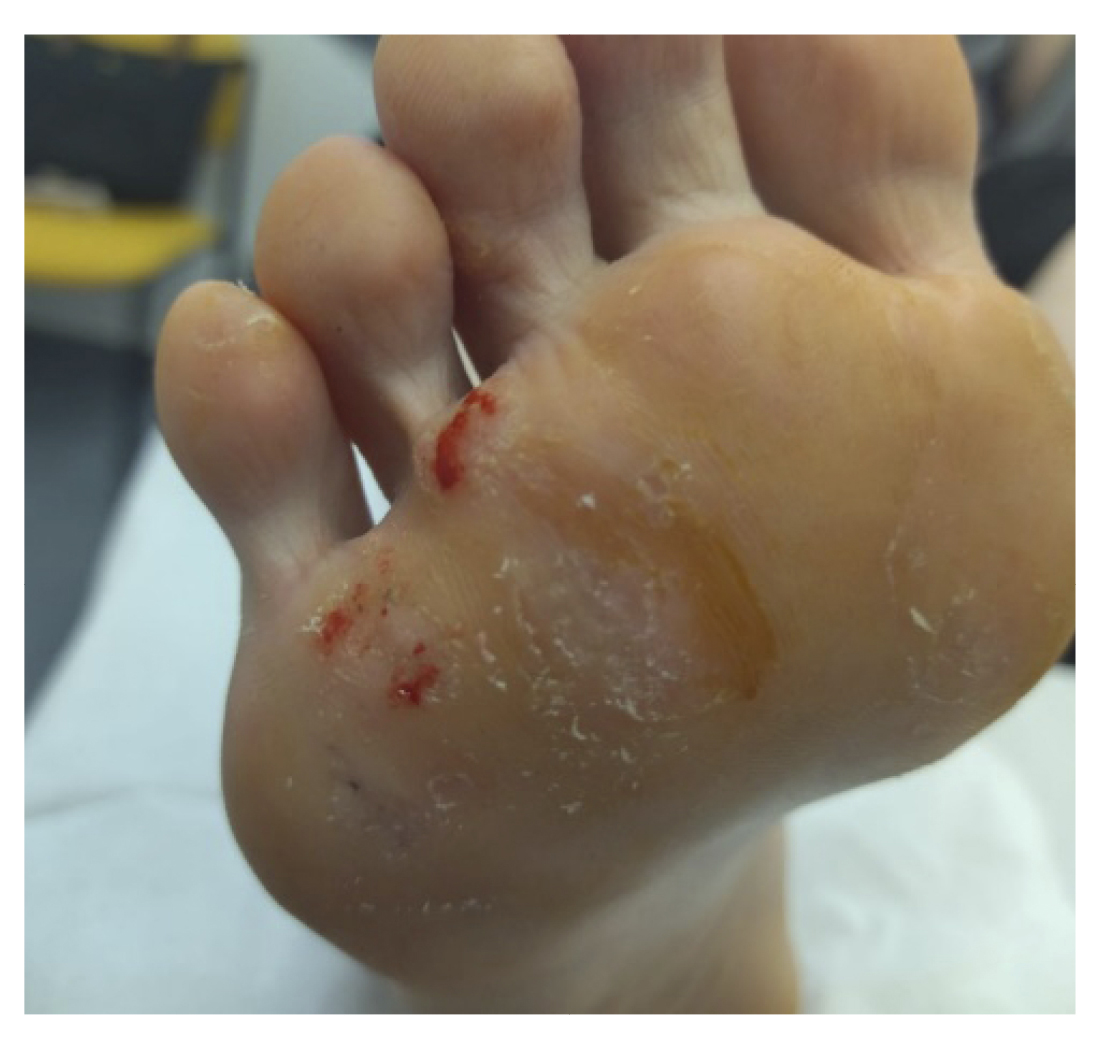
Figure 3. 1 week after starting treatment.
After two weeks (Figure 2. Week 4), the patient returned to the consultation and reported no pain, showing great satisfaction with the treatment and reporting that she had been able to resume the activities she performed before the onset of the lesions. We performed a delamination of all the lesions again, observing a clear improvement (presence of dermatoglyphs, mild hemorrhagic punctate but not very active) of the plantar lesions (Figure 4). At digital level, we saw that the digital lesions were resolved, maintaining the treatment in the metatarsal head area for two more weeks.
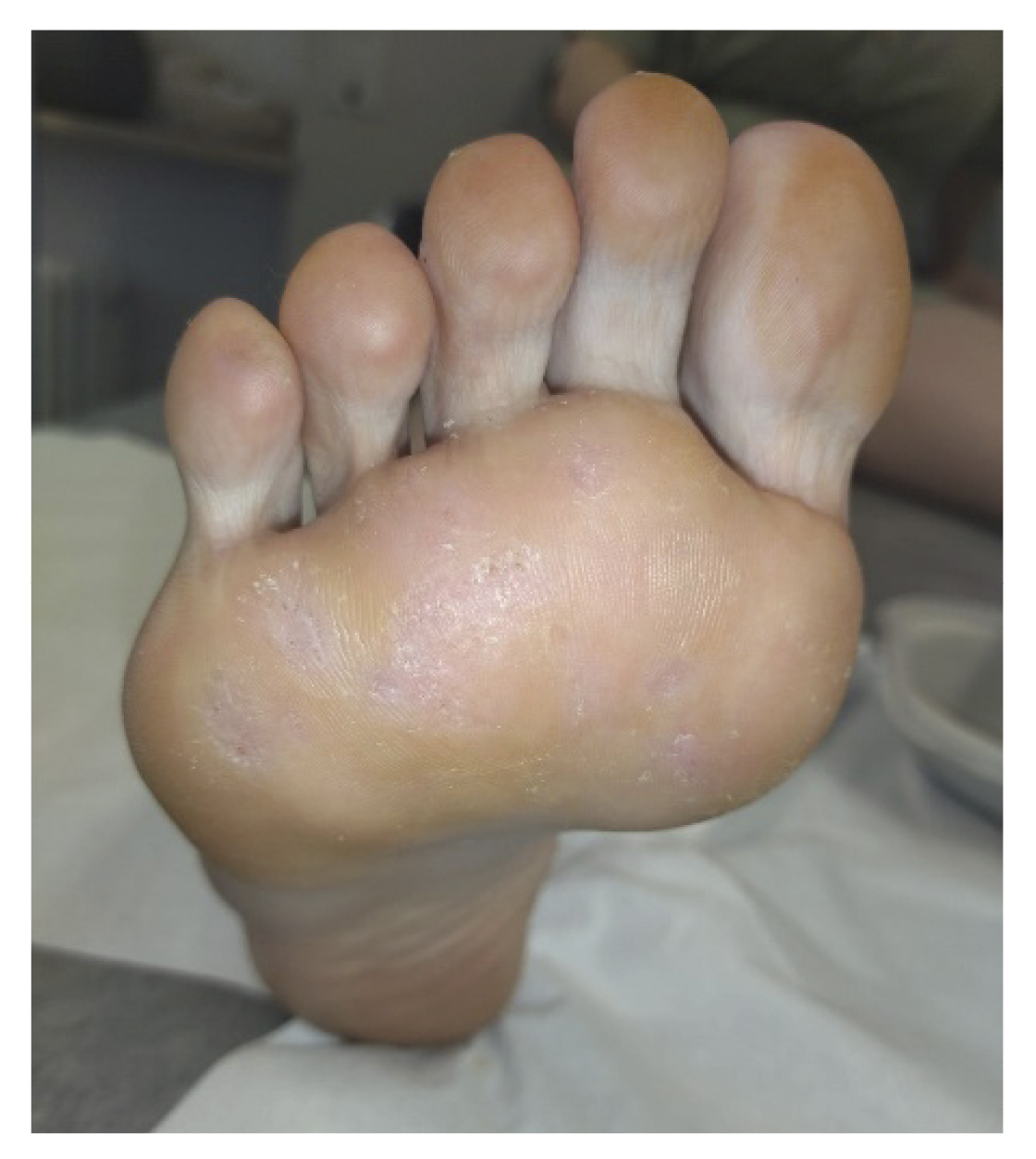
Figure 4. 3 weeks after starting treatment.
At two weeks (Figure 2. Week 6), in the new review, complete resolution of all lesions and absence of symptoms were observed (Figure 5). Despite the absence of lesions, due to the long evolution time of the lesions, the patient was instructed to apply the treatment at home for two more weeks until it was considered finished, and definitive discharge was not given, scheduling a review for one and a half months later.
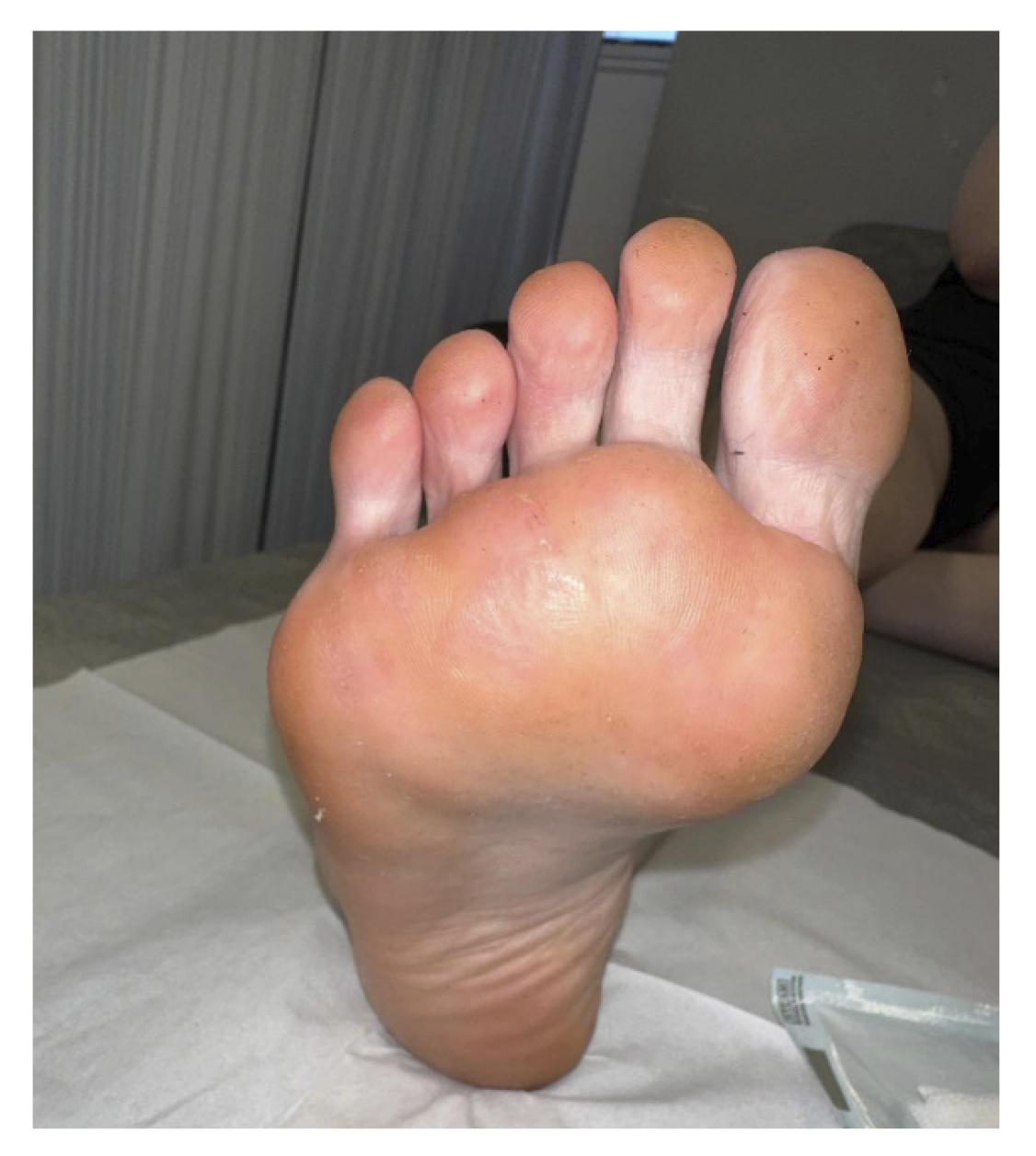
Figure 5. 6 weeks after starting treatment.
One and a half months later (Figure 2. Week 13), the patient returned for a follow-up visit. At that time, the patient continued without any discomfort, reporting a completely normal life. Upon inspection, no signs or symptoms of plantar warts were observed in any of the locations (Figure 6), so clinical discharge was performed, instructing the patient to return if she reported any discomfort again or observed any skin lesions.
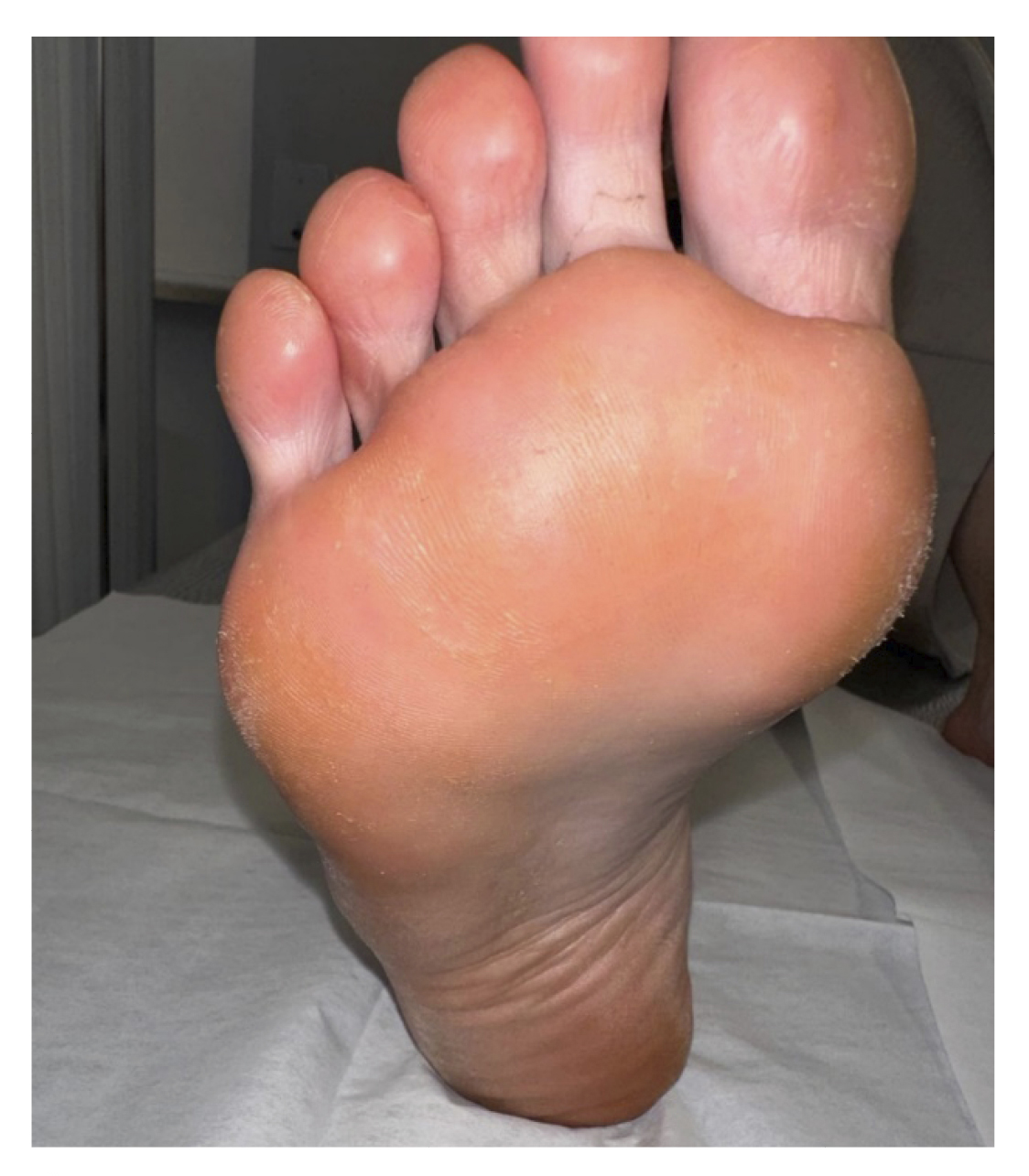
Figure 6. Review one and a half months after the resolution of the lesions.
Six months after the resolution of the lesions, a new consultation was conducted with the patient, who reported no discomfort and no reappearance of the lesions.
Discussion
The present paper presents a clinical case with complete resolution of recalcitrant plantar warts was achieved after 6 weeks of treatment with 5% Imiquimod cream. These lesions had been previously treated without improvement with multiple treatments.
Other case reports, such as those published by Mitsuishi et al. (14), have evaluated the efficacy of topical Imiquimod cream for the treatment of recalcitrant mosaic plantar warts. The first case published by Mitsuishi et al. (14) presented recalcitrant plantar warts of a 15-year history undergoing various treatments (cryotherapy, topical 5-Fluorouracil, intralesional bleomycin) without reporting favorable evolution. The patient applied 5% Imiquimod cream 3 times a week to the wart area, attending once a week for delamination of hyperkeratosis. After 16 weeks of treatment, total remission of all plantar warts was observed. The second case report presented various recalcitrant plantar warts, having been treated unsuccessfully with cryotherapy. Similarly, the patient applied 5% topical Imiquimod 3 times a week to the wart area, attending once a week for delamination of hyperkeratosis. After 14 weeks of treatment, total remission of all plantar warts was observed. After 3 months after finishing the treatment, both patients continued to show complete remission of the lesions. Therefore, the authors concluded that topical Imiquimod cream in combination with delamination of the thick stratum corneum of the skin is an effective option for the treatment of recalcitrant mosaic plantar warts in adults.
Another case report published by Yesudian et al. (15) showed the efficacy of Imiquimod for the treatment of recalcitrant plantar warts in a patient who presented with a 15-year history multiple plantar warts on both feet. The patient had been previously treated with the CPA formula and cryotherapy, without reporting any improvement. He was prescribed 5% Imiquimod cream 3 times a week for 8 hours without occlusion to apply to all plantar warts on the right foot, using the left foot as a control. At 4 weeks, a notable improvement in all warts on the right foot was observed, so the treatment was also started on the left foot. 8 weeks later, the plantar warts on both feet had completely disappeared. One year after finishing the treatment, the patient remained free of plantar warts. The authors concluded that Imiquimod is a good alternative for the treatment of recalcitrant plantar warts when previous treatments have not been successful.
A study conducted by López-Giménez et al. (16) evaluated the efficacy of Imiquimod for the treatment of recalcitrant plantar warts in 5 patients. Four of them had been on previous treatments without improvement. All patients were prescribed the application of 5% Imiquimod cream 3 times a week at night and without occlusion, applying 17% salicylic vaseline on the days when Imiquimod was not administered and attending every 2 weeks to remove hyperkeratosis mechanically with a scalpel. After an mean 5 weeks, complete healing of all warts was achieved, with no adverse reactions reported and very high patient satisfaction. They concluded that Imiquimod is effective, less injurious, and better tolerated for the treatment of recalcitrant plantar warts.
A controlled and randomized clinical trial conducted by Stefanaki et al. (17) aimed to study the efficacy of Imiquimod plus salicylic acid vs cryotherapy in children. Patients were randomly assigned to two groups, 50 people each. The first group received cryotherapy with liquid nitrogen every two weeks for a maximum period of 3 months. The second group was treated with 5% Imiquimod cream, applying it daily to the warts for 6 to 10 hours, five days a week, for a maximum of 3 months. After 3 months into treatments, no statistically significant differences were found between the groups treated with 5% Imiquimod with salicylic acid and the cryotherapy group, so the authors of this clinical trial concluded that Imiquimod combined with salicylic acid is as safe and as or more effective than cryotherapy for the treatment of plantar warts.
Currently, there are few publications in literature evaluating the efficacy of Imiquimod for the treatment of recalcitrant plantar warts, with only one published clinical trial. Therefore, this work addresses a novel and interesting topic of study, opening the door to future research.
However, this work has some limitations. It is not a clinical trial, and only one clinical case was included in the work, so no conclusive results were obtained.
In conclusion, Imiquimod may be an effective and safe drug and a good alternative for the treatment of recalcitrant plantar warts caused by HPV in adults and children. However, future high-level evidence studies such as controlled and randomized clinical trials should confirm its long-term safety and efficacy for the treatment of HPV plantar warts. The authors believe that future research will allow topical Imiquimod to be an indicated and preferred treatment for recalcitrant plantar warts.
Authors’ contribution
Study conception and design: MMS, SGO.
Data collection: MMS, SGO.
Analysis and interpretation of results: MMS, SGO.
Drafting, writing, and preparation of the manuscript: MMS, SGO.
Final review: MMS, SGO, JLLM, FJAA.
Ethical considerations
The patient voluntarily signed a consent statement for the use of her photographs and the publication of the details of her case.
Conflicts of interest
None declared.
Funding
None declared.
References
- Witchey DJ, Witchey NB, Roth-Kauffman MM, Kauffman MK. Plantar warts: Epidemiology, pathophysiology, and clinical management. J Am Osteopath Assoc. 2018;118(2):92-105.
- Vlahovic TC, Khan MT. The human papillomavirus and its role in plantar warts: A comprehensive review of diagnosis and management. Clin Podiatr Med Surg. 2016;33(3):337-53.
- García-Oreja S, Álvaro-Afonso FJ, García-Álvarez Y, García-Morales E, Sanz-Corbalán I, Lázaro Martínez JL. Topical treatment for plantar warts: A systematic review. Dermatol Ther. 2021;34(1):e14621. DOI: 10.1111/dth.14621.
- Navarro-Pérez D, García-Oreja S, Álvaro-Afonso FJ, López-Moral M, García-Madrid M, Lázaro-Martínez JL. Cantharidin-podophyllin-salicylic acid formulation as a first-line treatment for plantar warts? A case report with multiple plantar warts of human Papillomavirus biotype 27 and previous failed treatments. Am J Case Rep. 2022; 9(23):e937867. DOI: 10.12659/AJCR.937867.
- García-Oreja S, Álvaro-Afonso FJ, Tardáguila-García A, López-Moral M, García-Madrid M, Lázaro-Martínez JL. Efficacy of cryotherapy for plantar warts: A systematic review and meta-analysis. Dermatol Ther. 2022;35(6):e15480.
- Kumari P, Yadav D, Vijay A, Kumar Jain SK, Kumar M, Kumar R, et al. Falknor’s needling method as a potential immunotherapy in palmo-plantar warts. Indian J Dermatol Venereol Leprol. 2019;85:129.
- Alcalá Sanz J, Aranda Bolívar Y, Ahumada Bilbao J, Romero Prieto ME, Calvo Sánchez E. Cantaridina. Revisión bibliográfica como tratamiento de las verrugas plantares. Rev Esp Podol. 2011;22(3):107-11.
- Becerro de Bengoa Vallejo R, Losa Iglesias ME, Gómez-Martín B, Sánchez Gómez R, Sáez Crespo A. Application of cantharidin and podophyllotoxin for the treatment of plantar warts. J Am Pod Med Assoc. 2008;98(6):445-50.
- Salk RS, Grogan KA, Chang TJ. Topical 5% 5-fluorouracil cream in the treatment of plantar warts: A prospective, randomized, and controlled clinical study. J Drugs Dermatol. 2006;5(5):418-24.
- CIMA. Prospecto: información para el usuario: Imunocare 50 mg/g crema. Aemps.es. 2024. Disponible en: https://cima.aemps.es/cima/dochtml/p/78406/Prospecto_78406.html
- Skinner RB Jr. Imiquimod. Dermatol Clin. 2003;21(2):291-300.
- García-Oreja S, Álvaro-Afonso FJ, Sevillano-Fernández D, García-Morales EA, López-Moral M, Lázaro-Martínez JL. Does HPV biotype influence the characteristics and evolution of plantar warts? J Evid Based Med. 2024;17(1):10-2.
- BOE-A-2009-12002 Real Decreto 1015/2009, de 19 de junio, por el que se regula la disponibilidad de medicamentos en situaciones especiales [Internet]. Boe.es. Disponible en: https://www.boe.es/buscar/doc.php?id=BOE-A-2009-12002
- Mitsuishi T, Wakabayashi T, Kawana S. Topical imiquimod associated to a reduction of heel hyperkeratosis for the treatment of recalcitrant mosaic plantar warts. Eur J Dermatol. 2009;19(3):268-9.
- Yesudian PD, Parslew RA. Treatment of recalcitrant plantar warts with imiquimod. J Dermatolog Treat. 2002;13(1):31-3.
- López-Giménez MT. Five cases of recalcitrant plantar warts successfully treated with imiquimod 5% cream. Actas Dermosifiliogr. 2013;104(7):640-2.
- Stefanaki C, Lagogiani I, Kouris A, Kontochristopoulos G, Antoniou C, Katsarou A. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: A randomized study. J Dermatolog Treat. 2016;27(1):80-2.